What is decaf coffee? Simply put, decaffeinated coffee is coffee that has had most of its caffeine removed. For many, caffeine is the main reason they drink coffee—but for others, it can cause jitters, disrupt sleep, or trigger other sensitivities. That’s where decaf coffee comes in.
While your typical 8oz cup of coffee contains anywhere from 50 to 160 milligrams of caffeine, depending on how it’s brewed, a cup of decaf typically contains just 2 to 5 milligrams. That’s because around 97–99.9% of the caffeine is removed from decaf beans using various methods before the coffee is roasted. But how is decaf coffee made—and does it still taste good? Let’s explore the process, history, and innovations behind today’s best decaf coffee.
How to Decaffeinate Coffee: The Main Methods
How to decaffeinate coffee without ruining its flavor is a challenge that scientists and coffee producers have worked to solve for over a century. Caffeine is water-soluble, but so are many of the sugars, proteins, and acids that give coffee its flavor. The goal is to remove caffeine while leaving everything else intact.
There are three primary methods used to produce decaffeinated coffee:
1. Solvent-Based Decaf Coffee (Direct & Indirect)
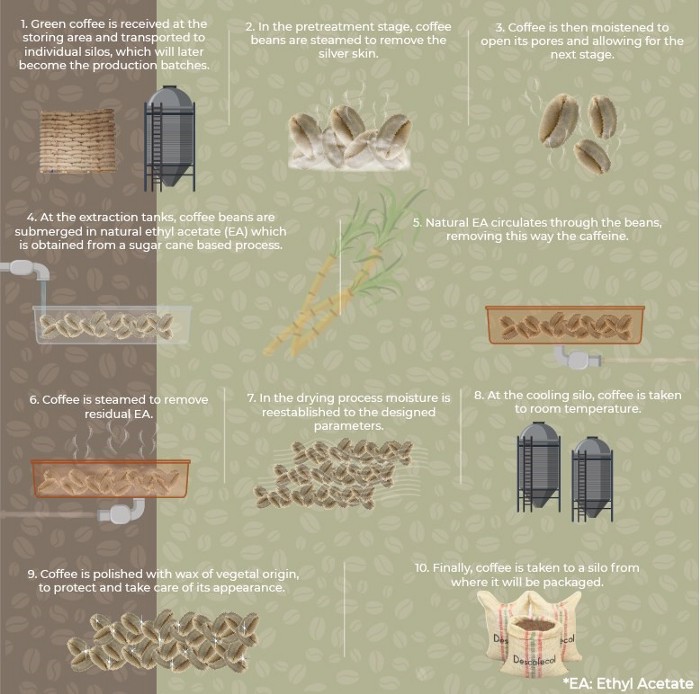
Descafecol ethyl acetate decaffeination (EA Decaf) process
Solvent-based methods are some of the oldest and most common. These processes use food-safe solvents like methylene chloride or ethyl acetate to extract caffeine.
- Indirect Solvent Process (aka European Method): Beans are soaked in hot water to extract caffeine and flavor compounds. The beans are then removed, and a solvent is added to the water to remove caffeine. The flavor-rich water is then reintroduced to the beans so they can reabsorb the good stuff.
- Direct Solvent Process: Beans are steamed and then soaked directly in a solvent solution. A standout version of this is the sugarcane decaf process, which uses naturally-derived ethyl acetate from fermented sugarcane. This method is popular in Colombia and preserves more of the coffee’s natural sweetness.
One example is Genuine Origin’s Sugarcane Decaf, decaffeinated with the Ethyl Acetate process. While the Ethyl Acetate used in the process may not necessarily be derived from sugarcane, our team looks for lots that are delightfully sugary and sweet.
2. CO2 Decaffeination (Carbon Dioxide Process)
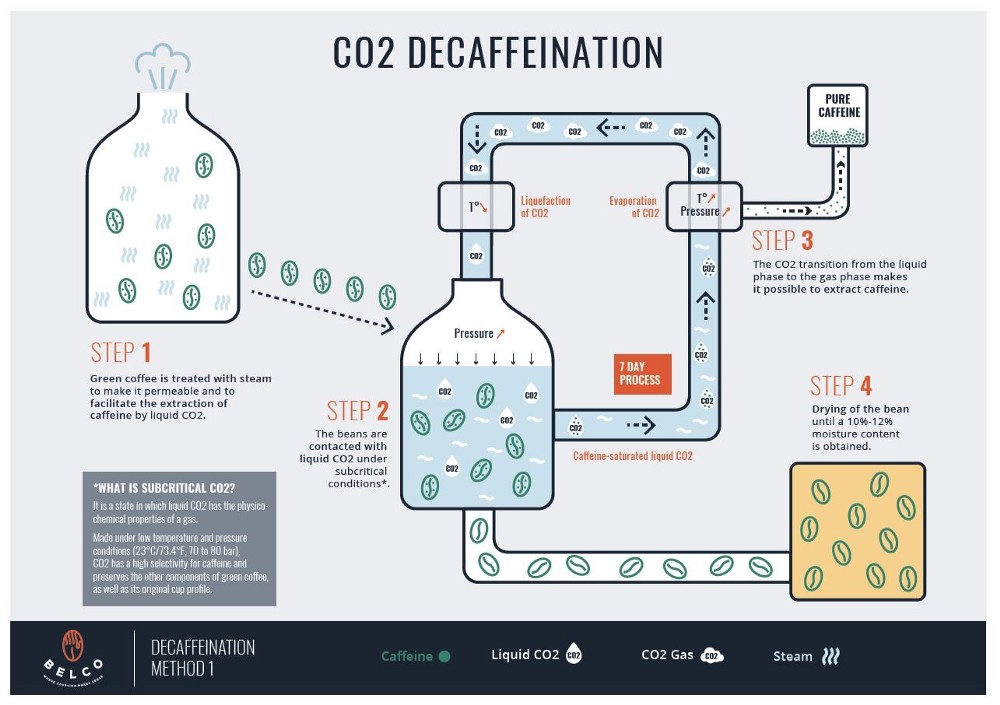
Belco decaf coffee processing method
The carbon dioxide decaf process uses liquid CO2 to selectively target and remove caffeine. It’s one of the most expensive and high-tech methods but ideal for retaining the complex flavors of high-quality beans. Beans are first soaked, then circulated with pressurized liquid CO2. The CO2 extracts caffeine and is later filtered and reused.
Because of its precision, this method is favored for preserving the original flavor of specialty-grade beans. If you’re looking for the best decaf coffee with minimal flavor loss, CO2 decaf is a solid bet.
3. Water-Based Decaffeination (Swiss Water & Mountain Water)
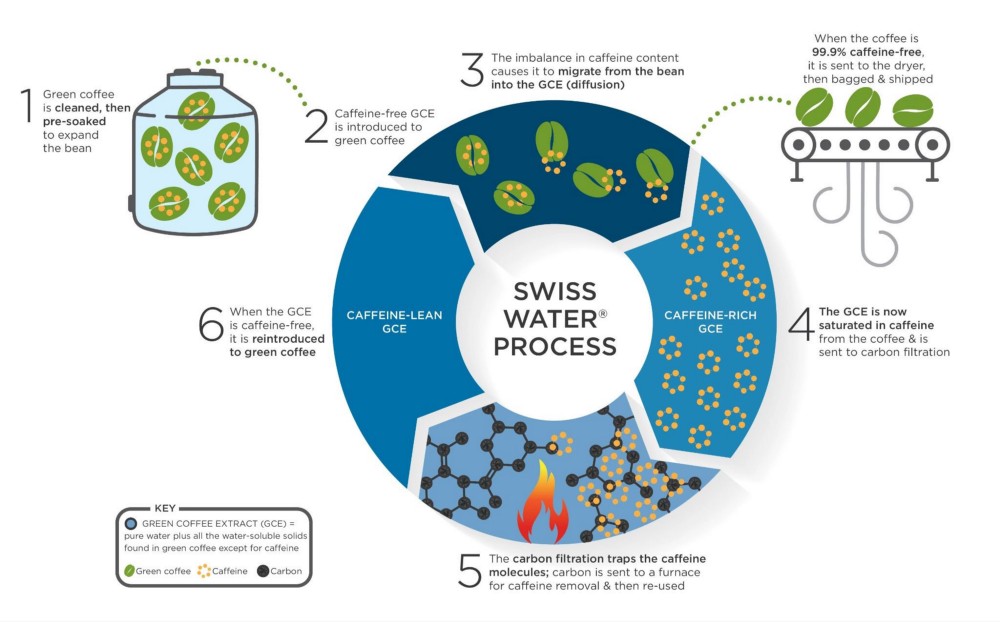
Swiss Water Coffee Decaffeination process
For those wondering, is decaf coffee bad for you?—these methods might offer peace of mind. Both Swiss Water Decaf and Mountain Water Decaf are chemical-free, making them a great option for those seeking organic or naturally decaffeinated coffee.
- Swiss Water Process: Developed in Switzerland and now used at a facility in Canada, this method uses pure water and a proprietary Green Coffee Extract (GCE) to remove caffeine. It results in 99.9% caffeine removal while preserving origin flavor.
- Mountain Water Process: Similar to Swiss Water but performed in Mexico using glacial water from Pico de Orizaba. Like Swiss Water, this method produces chemical-free decaf and retains certifications like organic or Fair Trade.
A great example is Genuine Origin’s Peru Amazonas Organic Swiss Water Decaf, a Swiss Water decaf with notes of milk chocolate, caramel, and green apple.
A Brief History of Decaffeinated Coffee
The first decaf coffee method was discovered by German merchant Ludwig Roselius in the early 1900s. He used brine and benzene, a now-banned and toxic solvent, to extract caffeine. Thankfully, decaffeination has come a long way, and today’s methods are much safer—and far more flavorful.
Modern decaffeination facilities in Canada, Mexico, and Colombia now use safer, more effective techniques to make sure your decaf delivers great taste with almost no caffeine in a cup of decaf coffee.
Does Decaf Coffee Have Caffeine?
Yes, decaf coffee does have caffeine, but only in trace amounts. The caffeine in decaf coffee typically ranges from 2 to 5 milligrams per cup, compared to 95 to 160 milligrams in regular coffee. If you’re asking, how much caffeine is in decaf coffee?, the answer depends on the method used, but the reduction is usually at least 97%.
That’s why it’s a great choice for anyone reducing caffeine intake without giving up their favorite morning ritual.
Is Decaf Coffee Bad for You?
No, decaf coffee is not bad for you. In fact, studies show that decaf may offer many of the same health benefits as regular coffee, including antioxidants and protective compounds, without the stimulating effects of caffeine. Decaf is a smart choice for pregnant people, those with heart conditions, or anyone who simply wants to sleep better. That said, in recent years, some researchers have raised concerns over the use of methylene chloride in decaffeination.
The Best Decaf Coffee Is Getting Better
Thanks to innovative decaffeination processes, decaf has shed its old reputation as bitter or flavorless. With options like Swiss Water, CO2, and sugarcane or Ethyl Acetate decaf, today’s best decaf coffee is flavorful, complex, and satisfying—without the jitters. After all, decaf coffee drinkers deserve delicious coffee, too!
At Genuine Origin, we’re proud to source high-quality decaffeinated coffee from partner producers using trusted and traceable methods. Whether you’re a coffee roaster looking for a delicious decaf option or a coffee lover curious about how decaf coffee is made, we’ve got you covered.
###
Find out more about Genuine Origin on our website — https://www.genuineorigin.com/decaf